Remdesivir wins U.S. approval as country’s first COVID-19 treatment drug
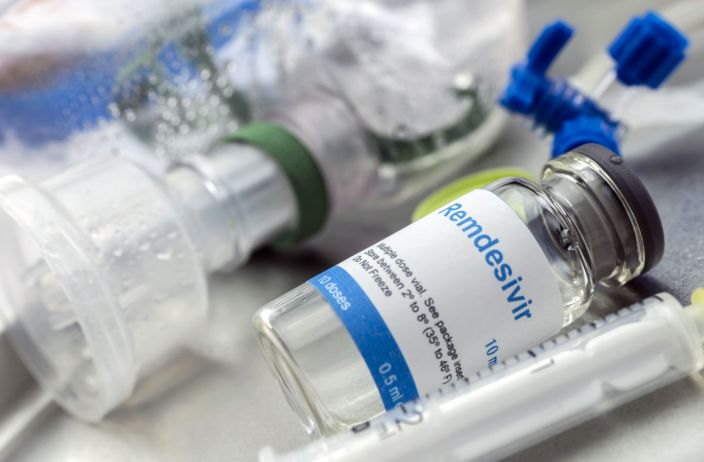
The U.S. Food and Drug Administration on Thursday approved Gilead Sciences Inc’s antiviral drug remdesivir for treating patients hospitalized with COVID-19, making it the first and only drug approved for the disease in the United States.
Remdesivir, given intravenously, was one of the drugs used to treat U.S. President Donald Trump during his bout with COVID-19.
The drug has been available under an FDA emergency use authorization since May, after a study led by the National Institutes of Health showed it reduced hospital stays by five days.
The FDA approval comes days after a study from the World Health Organization found no benefit of the drug in reducing early death or in preventing progression to serious disease among nearly 3000 COVID-19 patients. That study, however, did not include a placebo control and compared outcomes to standard of care. It’s also not clear how sick the patients in that study were and therefore how meaningful the results are.
A study in the New England Journal of Medicine included hints that people who receive the drug earlier in their disease may benefit more, and doctors are already studying whether people with mild symptoms but who don’t need to be hospitalized can be treated with remdesivir on an outpatient basis.
Remdesivir, which will be sold under the brand name Veklury, costs $3,120 for a five-day treatment course, or $2,340 for government purchasers such as the Department of Veterans Affairs. Shares of Gilead rose 4.3% in after-hours trading to $63.30.
“The formal FDA approval doesn’t change our (sales) estimates or outlook for remdesivir given it has already been branded standard-of-care prior to formal approval,” Raymond James analyst Steven Seedhouse said in a research note, calling the prescribing guidelines and approval “pretty much a best-case for Gilead,” given the WHO results questioning remdesivir’s benefits.