Phase 2 of a clinical trial for drug targeting kala-azar begins in Ethiopia

The second phase of a clinical trial for an oral drug targeting Kala-azar is underway in Ethiopia. Led by the non-profit research organization, Drugs for Neglected Diseases Initiative (DNDi) and its partners, the trial marks a significant advancement in combating the disease, also known as visceral leishmaniasis. It is the world’s second-deadliest parasitic killer after malaria.
Traditionally, treatment for kala-azar in Africa includes painful injections administered at hospitals over 17 days, with potentially life-threatening side effects. In contrast, the new oral medication under investigation in Ethiopia, known as LXE408, offers a safer alternative.
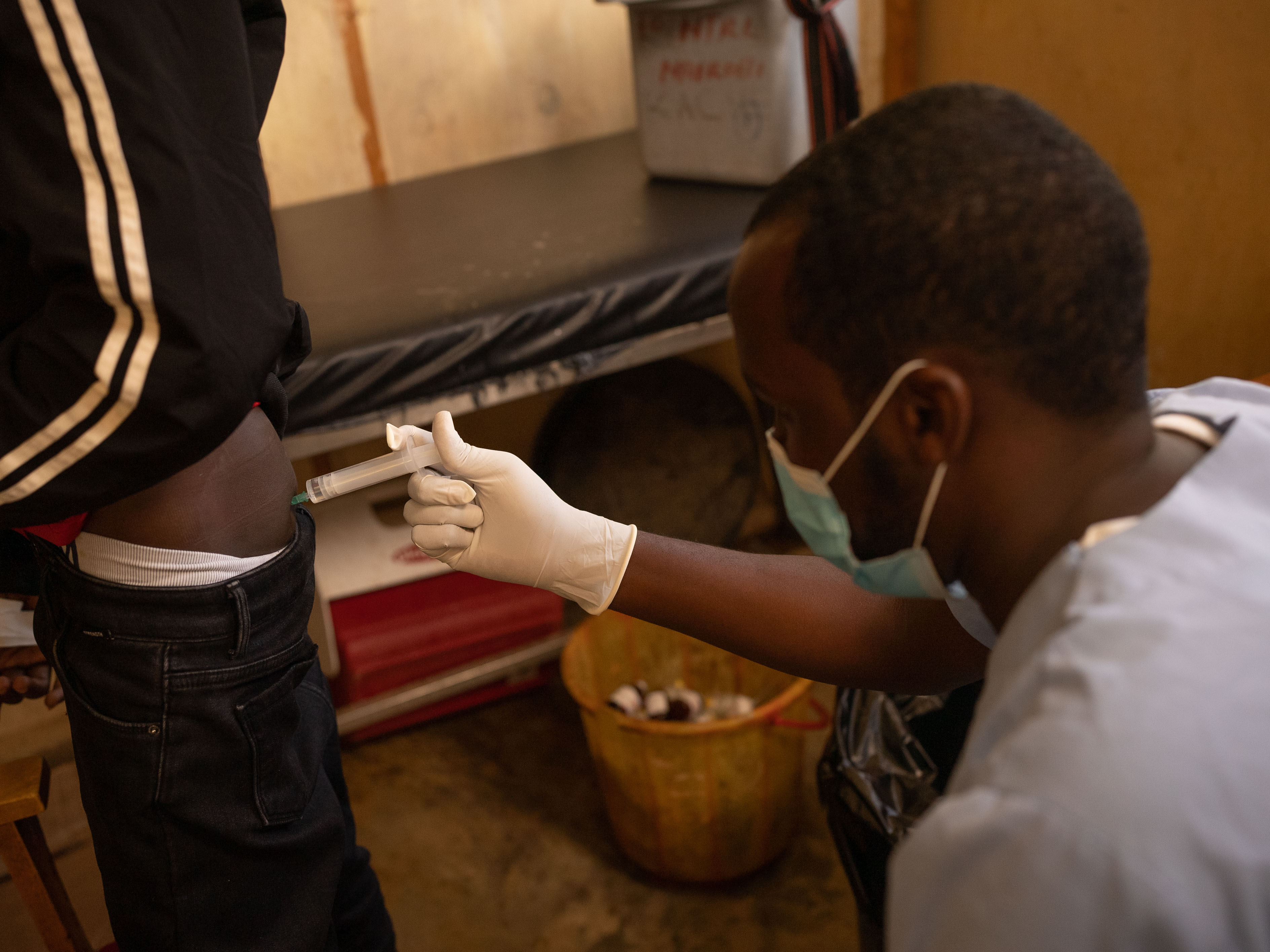
Traditionally, treatment for kala-azar in Africa includes painful injections administered at hospitals over 17 days, with potentially life-threatening side effects. In contrast, the new oral medication under investigation in Ethiopia, known as LXE408, offers a safer alternative.
The drug company, Novartis initially discovered LXE408. DNDi and Novartis initiated a collaboration and license agreement in early 2020 to jointly develop LXE408. Novartis has committed to distributing the drug on an affordable basis worldwide, if trial results are positive.

Dr Eleni Ayele, co-principal investigator of the clinical trial, emphasized the importance of this milestone.
“Current treatment options in Ethiopia have severe limitations,” Dr Ayele says. “They are potentially toxic, necessitate injections and cold-chain supplies, and require our patients to travel to faraway hospitals and be hospitalized for long periods.
Dr Ayele hopes this new oral treatment will be more efficient and less toxic and can be given to patients at the primary healthcare level, close to their homes. She says this would help patients access treatment earlier.
One billion people are at risk globally of contracting kala-azar. Eastern Africa has the highest number of cases. The infection spreads through the bite of infected sandflies and is endemic in 80 countries globally. It causes fever, weight loss, spleen and liver enlargement, and, if not treated, death.
Climate change exacerbates the disease’s spread, with an estimated 50,000 to 90,000 new cases annually, half affecting children under 15. The current treatment regimen in Ethiopia poses logistical challenges and potential toxicity risks.
Dr Fabiana Alves, director of the leishmaniasis program at DNDi, underscored the urgency of medical innovation to sustainably eliminate visceral leishmaniasis worldwide. The collaboration between DNDi and Novartis aims to develop LXE408 as part of this effort.
Funded by the European and Developing Countries Clinical Trials Partnership (EDCTP), the clinical trial in Ethiopia aligns with Eastern African countries’ strategies to eradicate visceral leishmaniasis sustainably amid climate and environmental changes.