Trials for most advanced experimental malaria vaccine to start in Malawi

A large-scale pilot test for the most advanced experimental malaria vaccine, Mosquirix, will begin in Malawi’s capital Lilongwe this week and in Kenya and Ghana next week.
The pilot targets 120,000 children aged two years and under to assess the effectiveness of the pilot vaccine and whether the delivery process is feasible.
Four successive doses must be administered on a strict timetable for the vaccine to work.
Malawi, Ghana and Kenya were selected because malaria rates are high and they have a long history of use of bed nets and other interventions.
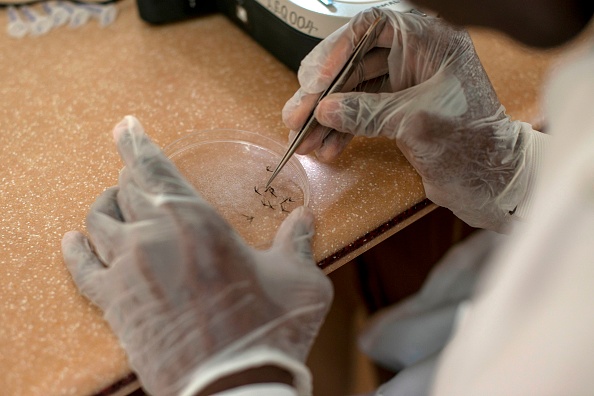
The drug was developed by British pharmaceutical company GlaxoSmithKline and the PATH Malaria Vaccine Initiative. It has taken more than three decades in development and almost $1 billion in investment.
The drug has had five years of clinical trials on 15,000 people in seven countries. It was approved for the pilot programme in 2015.
It is reported that malaria episodes reduced by 40 per cent in the trials.
Scientists say the vaccine is the furthest along in development and so far the most effective. However, it will not give full protection against malaria.
The World Health Organization (WHO) argues that the new vaccine adds a major new tool beyond mosquito nets, insecticides and drugs.
The WHO says the fight against malaria has also been complicated by mosquitoes building up resistance to some commonly used insecticides.
Malaria killed 435,000 people in 2017. Most of the victims of malaria were children under five in Africa.